Cheaper, better cancer cure
-
- from Shaastra :: vol 04 issue 06 :: Jul 2025
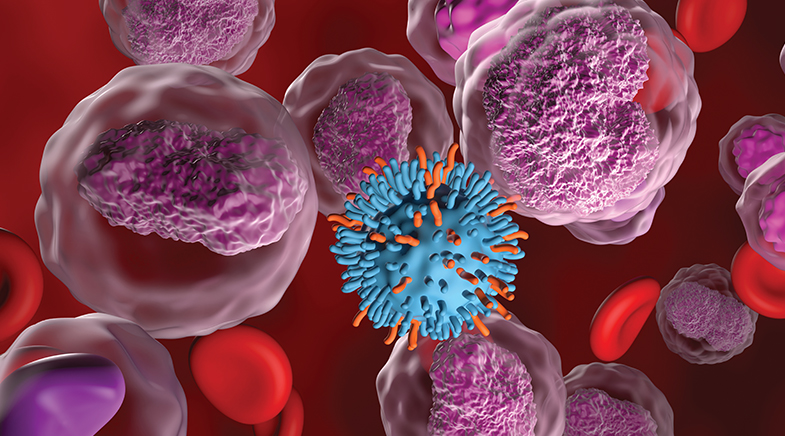
CAR-T therapies for cancer debut in India at a pocket-friendly cost.
Around two and a half years ago, a six-year-old boy faced a bleak future. Suffering from B-cell Acute Lymphoblastic Leukaemia (ALL), a type of blood cancer, he had undergone chemotherapy. But his aggressive cancer had returned. ALL, when it relapses, has a poor prognosis, with a 30-40% survival rate without a bone marrow transplant.
The second- and third-generation CAR-T cells were made for enhanced kill, improved amplification and persistence.
At the time, the Christian Medical College (CMC) Vellore was conducting a trial on the efficacy of the Chimeric Antigen Receptor T cell (CAR-T) therapy. The boy was enrolled for treatment under the trial, which was funded by the Indian Council of Medical Research (ICMR). Doctors at the hospital removed some blood from his veins and isolated his T cells, a type of white blood cells (WBC) that protect the body from infections and cancers. T cells have the ability, during their surveillance, to identify pathogenic cells, whether bacterial or those with oncogenic potential, and destroy them. Thus, not everyone gets cancer, even though several oncogenic cells circulate in the body. During cancers, these T cells lose their surveillance ability.
LIVING DRUG
CAR-T is a kind of immunotherapy or living drug. In this treatment, the patient's T cells are re-engineered by putting a genetic marker on their surface to help them identify cancerous cells. The marker is attached to the T cell with the help of a lentiviral vector, and it targets one of the specific proteins or antigens that get expressed in cancerous cells. The T cell is now a chimeric one and goes for the kill. For this trial, doctors used the vector developed by the German company Miltenyi Biotec. The chimeric cells were created at the CMC facility, reducing the time for transport.
The Cellogen team engineered T cells that could recognise two antigens. The ambitious therapy is positioned as a third- gen bispecific one.
Nine days after the boy's blood was taken, the doctors administered a dose of his own cells, now re-engineered to fight the cancer. Then, they waited. A month later, he was called back to the hospital for a blood test. The results were remarkable: the therapy was successful, and the cancer had gone into remission. "The child today is doing what children of his age should normally be doing. He remains in complete remission," says Vikram Mathews, Director of CMC Vellore and Professor of Clinical Haematology. Of the ten patients in that trial, aged between 6 and 59 years, the team lost only one patient, who did not respond to the low-dose treatment and died due to the progression of the disease. Even the oldest patient, now in his early 60s, recovered well despite not responding to three lines of therapy in the past, Mathews says. The hospital is now awaiting clearance from the Central Drugs Standard Control Organisation (CDSCO) to begin clinical trials for a new CAR-T treatment developed by NOIDA-based start-up Cellogen Therapeutics.
Initial CAR-T therapies entailed a one-on-one battle of a CAR-T cell with a cancerous cell. However, given the large numbers in which cancer cells were produced, there was a need to develop cells that could amplify as per the body's needs and persist, thus providing long-term protection. The second- and third-generation CAR-T cells, equipped with costimulatory domains on the surface, were tailored for enhanced kill, improved amplification and longer persistence.
Often, after the initial CAR-T infusion, the cancer returns with the expression of another antigen on the surface, which the engineered T cell cannot recognise. Doctors call such recurrences antigen escape mediatory lapses. The most common surface antigens are CD19, CD20, and CD22. So, the Cellogen team decided to engineer T cells that could recognise two antigens; thus, they are bispecific. The therapy is ambitious and positioned as a third-generation, bispecific one.
"The chances of the cancer escaping two antigens and a third antigen expression are rather negligible," says Gaurav Kharya, Co-founder of Cellogen Therapeutics and Director, Centre for Bone Marrow Transplant and Cellular Therapy, Indraprastha Apollo Hospitals, New Delhi. His team worked with the CSIR-Institute of Genomics and Integrative Biology (IGIB) to develop molecules loaded with these specifics and to create a vector capable of carrying such a heavy payload to the T cell. They developed and tested several constructs till they finally identified CD20-19 and CD22-19 as the best candidates, and got patents for both molecules. The trials, however, will proceed with the CD20-19 construct.

CAR-T therapy started in research labs several decades ago. Emily Whitehead was seven when she underwent a trial therapy in Pennsylvania, in the U.S., with CAR-T for relapsed leukaemia. Her recovery was a significant milestone in the therapy, which its creator Carl June's team had worked on for 20 years. In 2017, five years after her remission, the U.S. Food and Drug Administration (FDA) approved the first commercial CAR-T therapy. Whitehead remains cancer-free, and now advocates funding for scientific research.
"I was researching with the (U.S.) National Institutes of Health at the time, and I saw the CAR-T trials happening. I was keen to take this treatment back to India," says Tanveer Ahmad, another Co-founder of Cellogen Therapeutics. Kharya, seeking to create treatments for Indian patients at a cost that could be covered by insurance, found the right partnership with Ahmad, who is on a sabbatical as an Associate Professor at Jamia Millia Islamia University, New Delhi.
INDIA'S FIRST
The idea of providing accessibility and affordability to emerging cell and gene therapies to Indians had driven Rahul Purwar, Professor in the Department of Biosciences and Bioengineering at the Indian Institute of Technology (IIT) Bombay, to devise an indigenous CAR-T therapy. He was doing his postdoctoral studies at Harvard University, U.S., when research on CAR-T was gaining ground. Back home, he first began work on developing a lentiviral vector. Purchasing such vectors from other manufacturers contributed to the astronomical cost of CAR-T therapies. In 2018, he founded ImmunoACT, a company incubated at the IIT Bombay, and began clinical trials shortly at the Tata Memorial Centre, Mumbai.
In late 2023, the company got the clearance for its product NexCAR19 for treating specific relapsed or refractory B-cell Non-Hodgkin Lymphoma and B-cell Acute Lymphoblastic Leukaemia in patients whose standard therapies had failed. NexCAR19 is a second-generation molecule. It was the first — and indigenous — CAR-T therapy to be approved in India, and it was positioned as the world's most affordable such treatment. "The cost of treatment at present is ₹30-40 lakh, which is just one-tenth of the cost... (of such) a treatment in the U.S.," says Ankit Banik, a PhD scholar with Purwar at IIT Bombay, who has been associated with the research part of the project. In the U.S., for instance, one round of CAR-T could cost ₹6-7 crore.
Around 300 patients have been administered the NexCAR19 therapy so far, says Banik. An analysis of the data of the first 175 patients showed a 71% rate of complete remission among the lymphoma patients and 84% among leukaemia patients. Cytokine storms and neurotoxicity are the main side effects of the therapy, but only 6% needed intensive care to deal with these complications. Banik says the CAR molecule was "immunised" or designed to reduce side effects.
For NexCAR19 treatment, a patient's blood is sent to a centralised unit in Mumbai for manufacturing the CAR-T cells. With a streamlined logistical process in place, they manage a 29-day mean time, needle-to-needle, irrespective of geography within India.

In January 2025, CDSCO approved the second CAR-T therapy in India, called Qartemi. It has been developed by cell and gene therapy start-up Immuneel, which is financed by biotech entrepreneur Kiran Mazumdar-Shaw, cancer researcher-physician at Columbia University Siddhartha Mukherjee and U.S.-based doctor and life science therapies investor Kush Parmar. Qartemi uses a lentiviral vector licensed from Hospital Clínic de Barcelona and is approved for use to treat adult B-cell Non-Hodgkin Lymphoma. The CAR-T cells are manufactured at its facility in Bengaluru. It has already begun treating patients.
TRIALS AND CURES
From being a largely unknown and expensive line of treatment in India just a few years ago, CAR-T is now creating a buzz. More trials are in the pipeline, including a multi-centre Phase II clinical trial for Miltenyi Biotec and a paediatric trial for Qartemi. Currently, all therapies available and being tried in India are for B-cell leukaemia and lymphoma. Researchers, however, are exploring treatment for solid tumours, too.
"In blood cancer, the cancerous cells are in circulation, so it is easy for the CAR-T cells to kill them. The microenvironment of solid tumours makes the reach of T cells more difficult; there are lots of off-target effects," explains Sivaprakash K. Ramalingam, Associate Professor at IIT Kanpur's Molecular Medicine and Cellular Engineering Laboratory. He was with the IGIB earlier, where he helped clone and validate Cellogen's molecule. "We now have an ICMR-funded research to find therapies targeting triple-negative breast cancer," Ramalingam adds.
In China, a team has reported good results in Phase II clinical trials for CAR-T treatment for gastric tumours (bit.ly/solid-cancer). At IIT Bombay, Banik's research is on using another set of WBCs, called Natural Killer (NK) cells. Researchers worldwide are looking at ways to address the challenge of reaching solid tumours through immunotherapy. These include regulating the tumour's microenvironment, deploying CAR-T- and CAR-NK-based exosomes, and developing CAR constructs with special macrophages.
Currently, therapies available and being tried in India are for B-cell leukaemia and lymphoma, but researchers are also exploring treatment for solid tumours.
At present, CAR-T therapies are generally offered to patients who have relapsed after previous lines of treatment or who haven't responded to treatment (refractory cases). Some clinicians feel that success rates might improve if these are pushed up into an earlier line of treatment. "Patients who have gone through several lines of treatment tend to have a poorer response; their T-cells are already exhausted," notes Pankaj Malhotra, Head of Clinical Haematology and Medical Oncology at the Postgraduate Institute of Medical Education and Research, Chandigarh. He treated patients under Immuneel's Phase II multi-centre clinical trial, IMAGINE. CMC's Mathews adds that the results can be improved if CAR-T cells are manufactured at the point of care. "The needle-to-needle time is reduced, and the product is fresh, not frozen," he points out.
However, since CAR-T is still a new line of treatment, it is more expensive than standard chemotherapy, and has several issues that need resolution. The treatment is positioned either as a last resort or as a bridge between chemo and a transplant. "The therapy was initially envisaged as a one-time treatment, but given the problems of T cell persistence and antigen escape, we do have relapses," explains Sunil Bhat, who heads the Paediatric Oncology and Haematology Department at the Mazumdar Shaw Medical Center, Narayana Healthcity, Bengaluru. He was the Principal Investigator of the IMAGINE trial. "Of the 24 patients, around 40% had relapses, which we treated with bone marrow transplant."
Kharya hopes Cellogen's bispecific construct will lower relapse rates. Meanwhile, at Ahmad's lab in Jamia, the team has developed a fourth-generation molecule which could also lower relapse rates (New CAR-T promise).
Another issue with CAR-T therapy is the loss of natural immunity. The treatment targets B cells, which are the primary producers of immunoglobulins. "We need to be prepared for the journey beyond remission, especially the need for supportive care like intravenous immunoglobulins (IVIG)," notes Ruchira Misra, paediatric haematologist and oncologist and stem cell transplant specialist in Mumbai. Despite the post-treatment apprehension of a B-cell blast out, physicians say global data of the past decade indicate that most patients naturally recover some B cells and are weaned off from IVIGs within a couple of years. "From the IMAGINE trial, only one patient is on IVIG," Bhat says.
Despite these early pitfalls, CAR-T is on the march. In India, research is not just for better molecules, but for ensuring they remain pocket-friendly. While it might not be that silver bullet, it certainly does offer a silver lining of hope in the battle against cancer.
Also read
A 'gene therapy' revolution
Interview with Siddhartha Mukherjee
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH